
Introduction
This case study presents the successful implementation of an Automated Contract Approval Workflow for a Small Pharmaceutical company based in Nevada, USA. The primary goal was to reduce contract approval times by 50% and eliminate the need for printed paperwork
Business Scenario
The pharmaceutical company required an end-to-end contract lifecycle automation solution. This encompassed data and document collection, internal and external legal reviews, and final approval. The objective was to streamline contract management and enhance efficiency.
Medical, Legal and Regulatory (MLR) Department WorkFlow
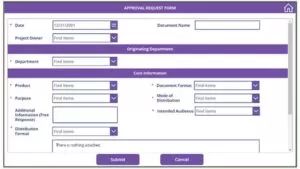
Stage – Form Submission
- A Submission Owner submits the application for approval of the materials to be shared with the State to the MLR Reviewers for their input before it is set out for distribution.
- Submission details are stored in a list.
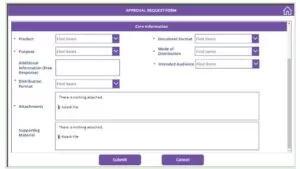
- There are 2 attachment options in the Form, one is to attach the prepared materials for approval, and another is to provide the supporting materials.
- The Supporting Materials and the Prepared Materials are stored in a folder in the Library with a custom code for each submission as the folder’s name.
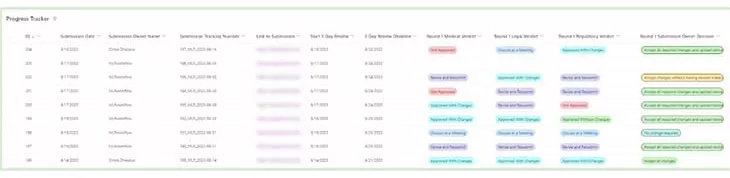
Stage – In-Progress Tracking
Upon submission, an entry is generated in the In-Progress Tracker, capturing essential information:
- Submission Date
- Submission Name
- Submission Tracking Number
- Submission Link
- Commencement of review
- Calculation of review completion date (business days)
- Timely reminders to ensure adherence to deadlines
Contract Request and Tracking App
Stage – Contract Submission
- A Business Owner initiates a contract, where they can place a request for Contract with an existing Supplier Entity or just create a new one in the process.
- After the submission has been made, email notifications are sent to respective teams for confirmation,
- A Business Owner can either submit a Contract or submit a Draft as well and come in on a later date with all information and submit the Contract,
- Once the contract has been placed, based on the department a Procurement Lead is assigned automatedly.
Stage – Contract Tracking Grid
- All the Contracts that have been submitted will come up in the grid for the Procurement Leads.
- Submission Owner can also review the submitted information but cannot change anything after submitting the contract.
- Contracts that are either “Completed” or “Cancelled” appear at the end of the Grid.
- Procurement Leads can change the required status from the Grid, without having to go in the request and update manually.
- Based on predefined conditions the status columns would have the highlighted border as specified.
Stage – Review and Collaboration
- The Grid can be accessed by the Procurement department as well as other defined users which can update only certain statuses as requested.
- Once the contract has been completed, email notifications are sent out to users for confirmation.
- Multiple departments can come to one place to collaborate and work on the contracts.
Project Details
| Customer | Small Pharmaceutical, USA, Nevada |
| Domain | Pharma |
| Division/ Department | Legal |
| Engagement Mode |
|
| Tools and Technology | Power Automate, SharePoint Online, Office 365 |
| Development Environment | Windows 10 |
Results
The implementation of the Automated Contract Approval Workflow resulted in a completion of contract refinement and approval in half the time, resulting in savings in time, effort and faster execution in the pharmaceutical company. By embracing digital tools and automation, the company achieved its goal of reducing contract approval times while eliminating the need for printed paperwork.
Conclusion
This case study showcases how the adoption of automated workflows using modern tools and technologies can lead to significant and noticeable efficiency gains and process improvements. The pharmaceutical company’s commitment to enhancing its contract management process demonstrates the potential benefits of embracing digital transformation in various industries.
Want to talk?
Drop us a line. We are here to answer your questions 24*7.



